Hemophilia Gene Therapy Market to Hit USD 3.06 Billion by 2032
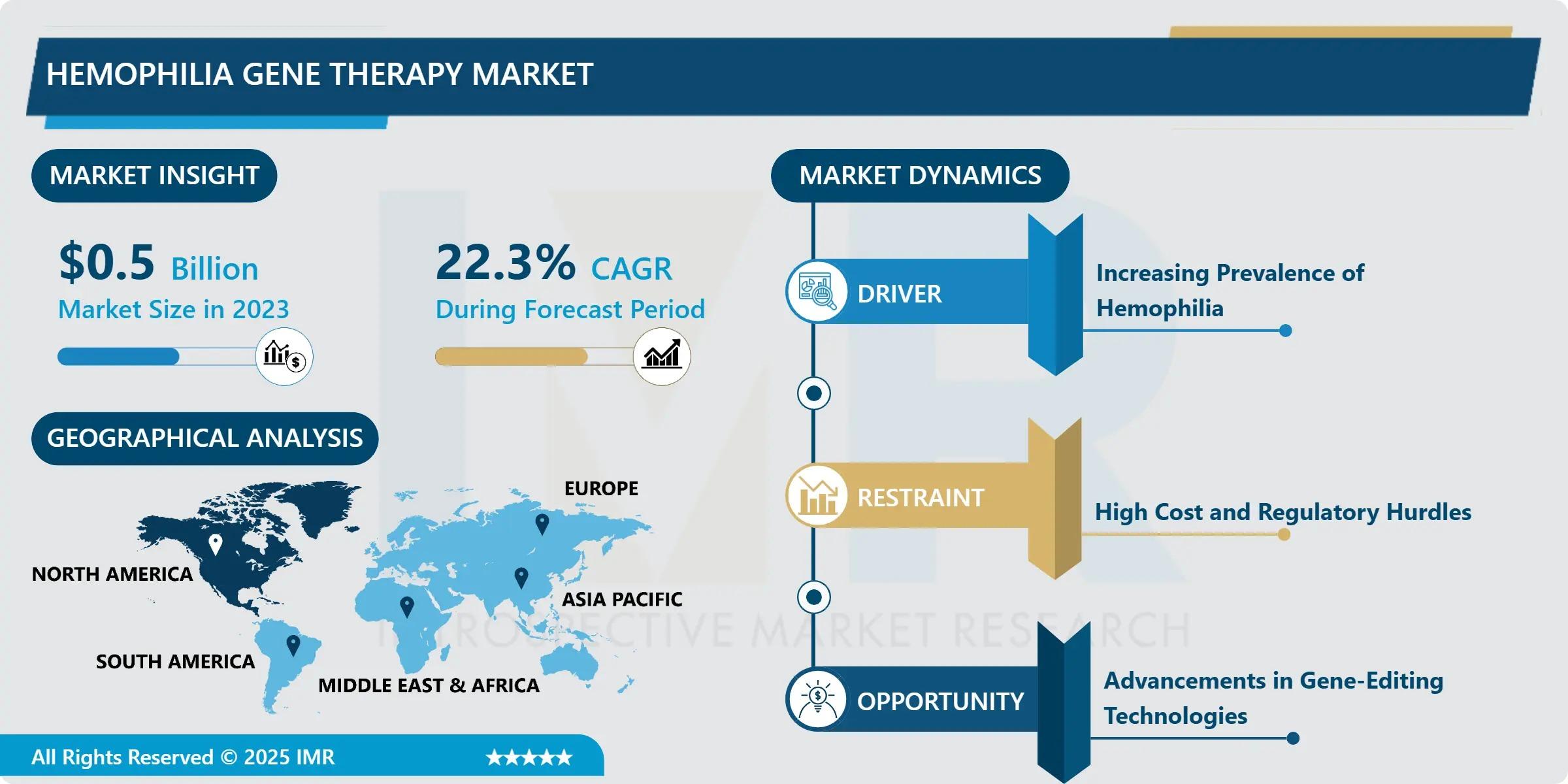
“According to a new report published by Introspective Market Research, Hemophilia Gene Therapy Market by Therapy Type, Indication, and End User, The Global Hemophilia Gene Therapy Market Size Was Valued at USD 0.5 Billion in 2023 and is Projected to Reach USD 3.06 Billion by 2032, Growing at a CAGR of 22.3% from 2024–2032.”
The Hemophilia Gene Therapy Market is witnessing rapid advancement as innovative genetic treatments redefine the management of hemophilia, a rare inherited bleeding disorder. Unlike conventional replacement therapies that require frequent administration of clotting factors, gene therapy offers a long-term or potentially curative approach by introducing functional genes to restore clotting factor production. This paradigm shift is driving strong interest from healthcare providers, patients, and biopharmaceutical companies worldwide.
Gene therapy for hemophilia primarily targets Hemophilia A and Hemophilia B, using viral vectors to deliver functional copies of the deficient gene. These therapies significantly reduce bleeding episodes, improve patient quality of life, and minimize long-term complications associated with repeated infusions. The growing success of clinical trials and regulatory approvals is accelerating market adoption.
The market is further supported by increasing investment in rare disease research, favorable regulatory incentives, and rising awareness of advanced treatment options. As healthcare systems prioritize innovative therapies with long-term economic benefits, hemophilia gene therapy is emerging as a transformative solution in hematology.
Market Segmentation
The Hemophilia Gene Therapy Market is segmented into Therapy Type, Indication, and End User.
By Therapy Type, the market is categorized into AAV Vector-Based Therapy, Lentiviral Vector-Based Therapy, and Others.
By Indication, the market is categorized into Hemophilia A and Hemophilia B.
By End User, the market is categorized into Hospitals, Specialty Clinics, and Research & Academic Institutes.
Growth Driver
The primary growth driver of the Hemophilia Gene Therapy Market is the increasing demand for long-term and potentially curative treatment solutions. Traditional clotting factor replacement therapies require lifelong, frequent infusions and are associated with high treatment costs and patient burden. Gene therapy addresses these challenges by offering sustained therapeutic effects with a single administration. Rising clinical success rates, strong efficacy outcomes, and growing patient preference for one-time treatments are significantly accelerating adoption. Additionally, supportive regulatory frameworks for orphan drugs and rare diseases are encouraging faster approvals and commercialization of gene therapies globally.
Market Opportunity
A major market opportunity lies in the expanding pipeline of gene therapy products and increasing investment from biopharmaceutical companies. Ongoing research efforts aim to improve vector efficiency, safety profiles, and durability of gene expression, creating opportunities for next-generation therapies. Emerging markets with improving healthcare infrastructure also present untapped growth potential. Furthermore, collaborations between biotech firms, academic institutions, and healthcare providers are expected to accelerate innovation, reduce development timelines, and expand patient access, positioning hemophilia gene therapy as a key growth area within precision medicine.
Detailed Segmentation
Hemophilia Gene Therapy Market, Segmentation
The Hemophilia Gene Therapy Market is segmented on the basis of Therapy Type, Indication, and End User.
Therapy Type
The Therapy Type segment is further classified into AAV Vector-Based Therapy, Lentiviral Vector-Based Therapy, and Others. Among these, the AAV Vector-Based Therapy sub-segment accounted for the highest market share in 2023. This dominance is attributed to its high transduction efficiency, proven safety profile, and widespread use in advanced clinical trials. AAV-based therapies have demonstrated long-term expression of clotting factors, reducing bleeding frequency and improving patient outcomes, making them the preferred choice among developers and clinicians.
Indication
The Indication segment is further classified into Hemophilia A and Hemophilia B. Among these, the Hemophilia A sub-segment accounted for the highest market share in 2023. This is due to the higher prevalence of Hemophilia A globally and the strong pipeline of gene therapies targeting factor VIII deficiency. Continuous advancements in vector design and dosing strategies have significantly enhanced therapeutic efficacy for Hemophilia A patients.
Some of The Leading/Active Market Players Are-
• Roche Holding AG (Switzerland)
• BioMarin Pharmaceutical Inc. (USA)
• CSL Behring (Australia)
• Pfizer Inc. (USA)
• Sangamo Therapeutics Inc. (USA)
• UniQure N.V. (Netherlands)
• Spark Therapeutics Inc. (USA)
• Takeda Pharmaceutical Company Limited (Japan)
• Bayer AG (Germany)
• Freeline Therapeutics (UK)
• Novo Nordisk A/S (Denmark)
• Sanofi S.A. (France)
• Regeneron Pharmaceuticals Inc. (USA)
• Octapharma AG (Switzerland)
• and other active players.
Key Industry Developments
News 1:
In June 2023, a leading biopharmaceutical company announced positive Phase III clinical trial results for its gene therapy targeting Hemophilia B.
The study demonstrated sustained factor IX expression with a significant reduction in annual bleeding rates, reinforcing confidence in gene therapy as a long-term treatment solution and supporting regulatory submission plans.
News 2:
In November 2024, a global regulatory authority approved a novel gene therapy for Hemophilia A.
This approval marked a major milestone for the industry, enabling broader patient access and encouraging further investment in next-generation gene therapy platforms for rare bleeding disorders.
Key Findings of the Study
• AAV vector-based therapies dominate the market due to proven efficacy
• Hemophilia A represents the largest indication segment globally
• North America leads the market with strong regulatory support
• Rising demand for curative treatments is driving market growth
More Info:- https://introspectivemarketresearch.com/reports/hemophilia-gene-therapy-market/
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the Hemophilia Gene Therapy Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global Hemophilia Gene Therapy industry.
📞 Contact Us
Introspective Market Research Pvt. Ltd.
Phone: +91-91753-37569
Email: sales@introspectivemarketresearch.com
Web: www.introspectivemarketresearch.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Oyunlar
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness